A Kiambu-based pharmaceutical firm, Universal Corporation Limited, risks facing a legal suit for exposing the lives of HIV patients to danger by selling ARV drugs with compromised safety standards.
The Pharmacy and Poisons Board (PPB) recalled the drugs in a white bottle with black writings on the side and the wordings ‘Universal Corporation Ltd’.
According to Chief Executive Officer of PPB Dr. Fred Siyoi, they questioned the quality of the drugs due to discoloration of the induction seal and the tablets changing colour as well as the bottle covers.
The recall was on all Tenofovir/Lamivudine/Dolutegravir 300/300/500 mg (TLD) tablets manufactured by Universal Corporation Limited.
The National Empowerment Network of People living with HIV/AIDS in Kenya (Nephak) is, however, concerned that some of its members received the compromised medicine, but in different packages.
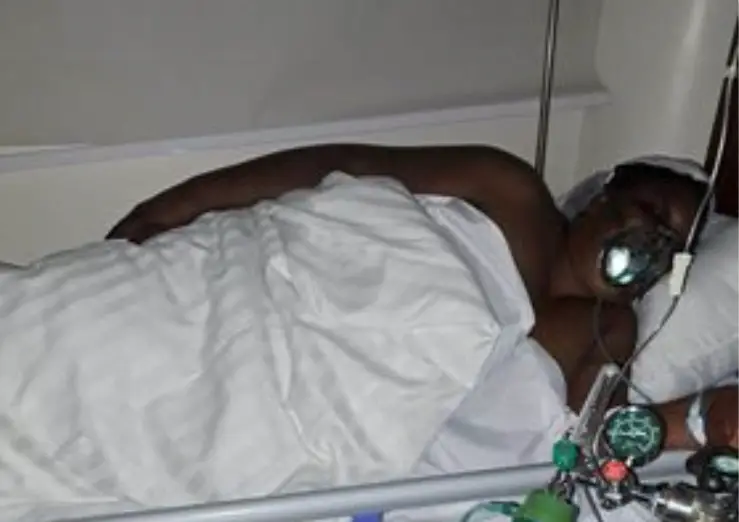
“When you tell our members to return their doses when some hospitals don’t offer a replacement, then you want them to die. We are alive because of the drugs,” Nelson Otwoma said.
He questioned the fate of users who have been adhering religiously to contaminated drugs.
“This is joking with people’s lives! In as much as we are doing our best to return the drugs, the damage is already done. There are people who have taken it for months. What will happen if they withdraw and embark on drugs from other manufacturers?” he asked.
The drugs were ordered by the Kenya Medical Supplies Authority (KEMSA) through UCL which is pre-qualified by the World Health Organization (WHO) and paid for by the global fund.
Acting director-general for health, Dr Patrick Amoth said that there is a sufficient national stock of TLD from other brands that could last the country for the coming six months, adding that only the batch manufactured by UCL is affected.
Perviz Palu Dhanani is the Founder and Managing Director of Universal Corporation Limited currently operating in over 22 countries.
The firm was established in Kenya over 20 years ago and is currently manufacturing more than 100 formulations of human medicines.